Testing & Validation
When a business implements a new computer-based system or needs to validate an existing system, they will need to provide evidence to show that the system does exactly what it is designed to do, that it is consistent, accurate, reliable, traceable and secure.
To achieve this, there are a number of requirements that need to be completed before a system is considered fully validated.
SoftNLabs can help you achieve a successful validation project.
SoftNLabs can be of assistance
by constructing or reviewing the following:
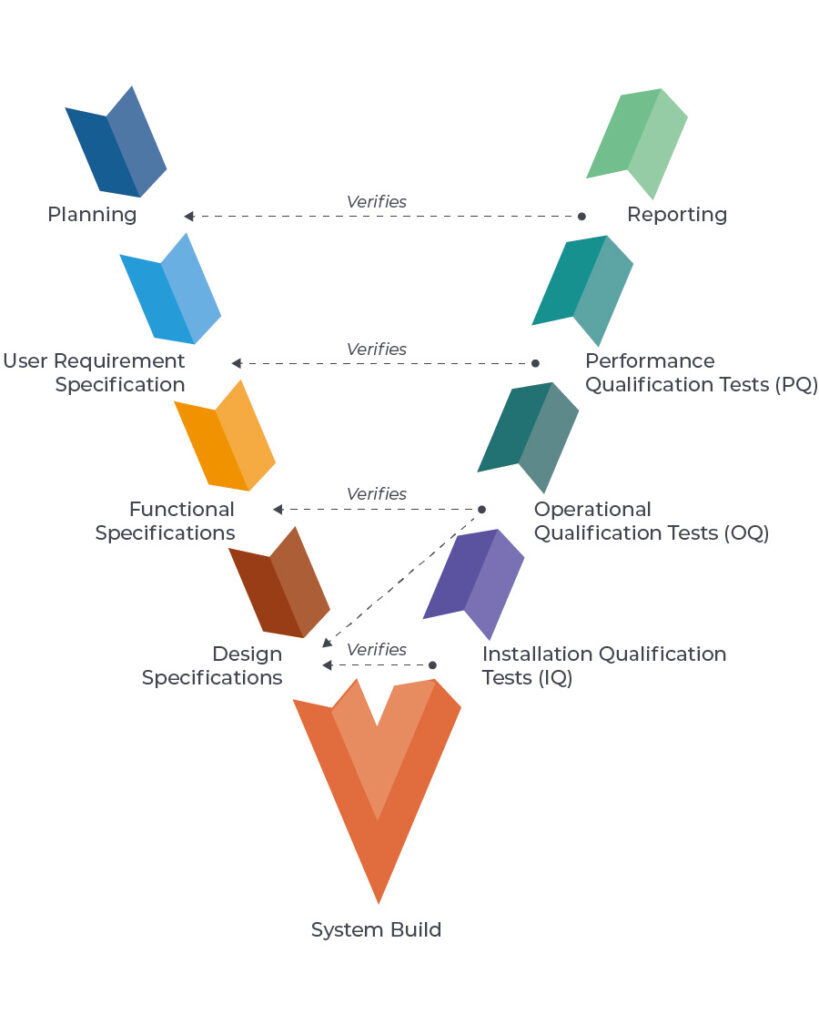
One example methodology for system validation is following the V Diagram, as shown in the diagram below:
There are a number of pitfalls that you need to be aware of when conducting a computer system validation project.
Below are some examples of common pitfalls that should be avoided:
-
Poor planning:
Poor planning can lead to a poorly run validation project or even an unsuccessful outcome. It is important to create a good validation plan (VP).
-
Poorly defined requirements:
Inadequately or poorly defined user requirements specification (URS) / functional specifications (FS) can result in the system not being able to be verified it is working as intended. A clear and precise set of requirements is essential to allow for the accurate testing of the system and to show it does what it is intended to do.
-
Lack of resources:
Often, day to day business needs of members of the project team can take precedence over the project. This can have an impact on the time dedicated to the project, leading to delays or the project being abandoned. The project team should ideally have dedicated and experienced members.
-
Time wasting:
To avoid wasting time and cost on low value testing, it is important to conduct a risk assessment to determine the level and aspects of the system that require testing. Focus should be on what is achievable and practical to satisfy regulatory and quality compliance. It is important not to get bogged down in the “Nice to haves”.
-
Inadequate / Excess documentation:
It is essential to know what documentation is required for the validation of the system. Too little will not satisfy quality and compliance. Too much can lead to an overrun and costly project.
We have the pleasure to work with
the following industries:

pharmaceutical
food & beverage

chemical & petrochemical

laboratory

other activities
Any project?
Our team is available for you.